Industrial Dehumidification Part 1:
by William G. Acker |
Articles | ||
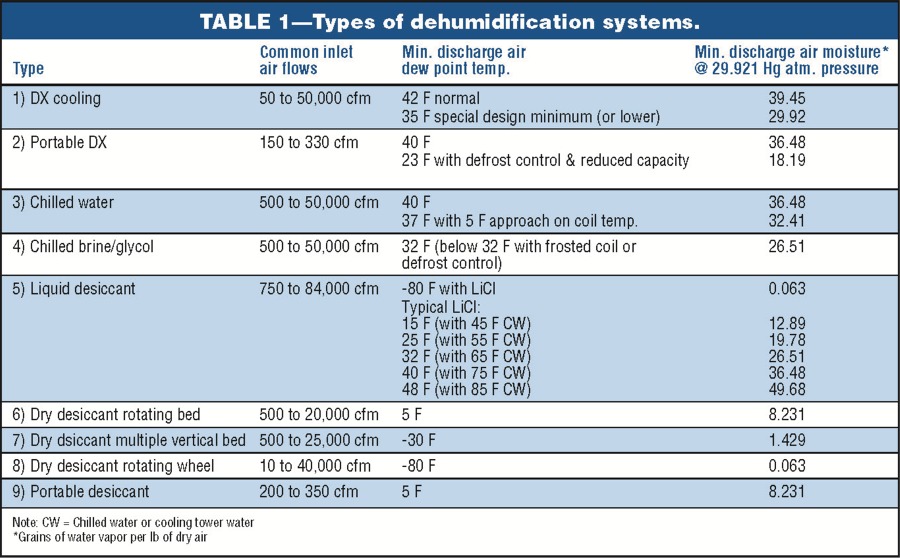 Types of Dehumidification SystemsSome of the types of equipment and their moisture-removal capabilities are illustrated in Table 1. Moisture can be removed from the air by cooling it below the dewpoint temperature so condensation occurs by air-to-air heat exchangers, which bring in dryer outside air, or by chemical methods. Chemical dehumidification is carried out through the use of sorbent materials, which are solids or liquids that can extract moisture from the air and hold it. There are two classifications of sorbents:• Adsorbents—which do not experience a phase change. Moisture is deposited on the surface of the dry desiccant. Most adsorbents are solids. • Absorbents—which change physically, chemically, or both during the sorption process. Most absorbents are liquids or solids that become liquid as they absorb moisture. Portable DehumidifiersWhen most people think of portable dehumidifiers, they think of equipment that is used in the home, but this is no longer true. These systems are being used in many commercial and industrial applications. A few examples of their uses are: indoor pools, cleaning and restoration, locker rooms, pump stations, libraries, restaurants and bars, film and tape storage, bakeries, well houses, and canning plants.The types of portable equipment available are direct expansion (DX) reheat, dry desiccant, and air-toair heat exchangers—to name a few. The water-removal capacities of these systems at ANSI B149.1 inlet air conditions (80 F and 60 percent RH) are as follows: • cDX reheat: 0.65 to 4.35 lb per hr; 1.88 and 12.50 gal per day, respectively. • Dry desiccant: 6.26 lb per hr; 18 gal per day. • Air changers: 15.30 lb per hr; 44 gal per day. (Building air is exhausted through an air-to-air heat exchanger that brings in dry outside air. Removal capacity is based on outside air at 0 F and 60 percent RH.)  Table 2 is a summary of more than 30 portable dehumidifiers reviewed for this article. The table illustrates both the waterremoval capacity and the operating cost of each system. Notice that the energy- inefficient systems use up to four times more energy than the efficient systems. Table 3 presents the variation of water-vapor removal and the operating cost at various inlet air conditions. This table was based on one of the energy- efficient models. Some DX-reheat units will begin to form frost on the cooling coils when the inlet air dry bulb goes below 60 to 65 F. If the unit has frost control, it will experience significant capacity loss due to the frost-control operation. Two typical approaches to frost control are listed below: • A temperature-sensing thermostat diverts the hot refrigerant gas through the evaporator coil until the ice is melted. • An automatic de-ice sensor shuts the compressor off when evaporator-coil temperature approaches freezing. The fan continues moving warm air across the coil to defrost it.  Large Industrial DehumidifiersThis section of the article is a detailed review of three common types of industrial dehumidifiers: direct expansion, dry desiccant, and liquid desiccant. Each system has an air intake of 30,000 cu ft per min at 70 F and 56 grains of water vapor per lb of dry air. Also, each system was required to dry the air down to 35 or 36 grains of water vapor per lb of dry air. I worked with several dehumidification companies to size the systems and determine all energy consumption. Using the brake horsepower provided by the companies, I sized the motors and calculated the electrical consumption (KW per hr) illustrated in this article. Operating costs were then developed using $0.06 per KWH for electricity and $5.00 per 106 Btu for natural gas. The operating costs were then developed into operating cost per 1000 lb of water vapor removed. In some cases, I left out proprietary information on the systems at the request of the manufacturers.Each industrial dehumidifier includes detailed air- and water-vapor mass-flow analysis, psychrometrics, thermodynamics, and adiabatic mixing (Figs. 1 to 3) to help the readers understand the energy flows. The psychrometrics and thermodynamics used in the diagrams follow the principals of Zimmerman and Lavine. The airand water-vapor flows are also illustrated in acfm (actual cu ft per min) and the dehumidification industry dscfm (dry standard cu ft per min). I used dscfm to avoid confusion with the fan industry scfm. Cooling-based DehumidificationMoisture can be removed from the air by cooling the air below its dew-point temperature. This can be achieved through the following systems:• Chilled water, glycol, or brine coil system 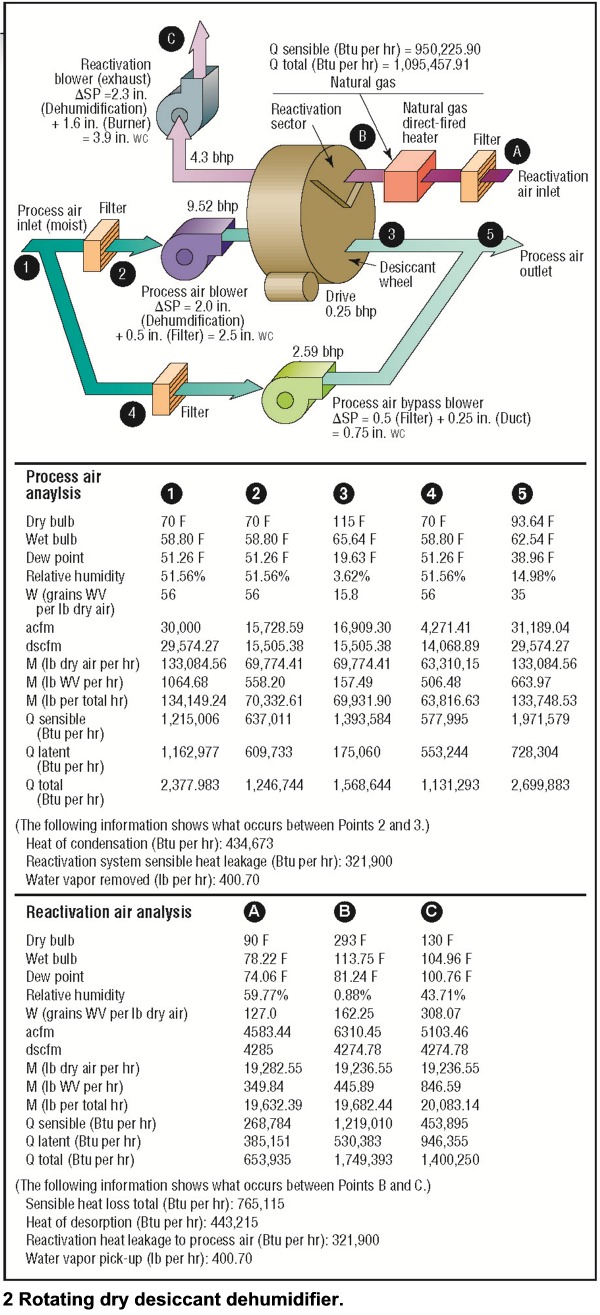 • DX cooling coil system • Chilled water air-washer system The first two systems accomplish dehumidification by passing the air through a cooling coil with a coil-surface temperature below the dew point of the air. Water vapor condenses on the coil surfaces. The amount of moisture removal depends on how cold the air can be chilled. The lower the temperature, the drier the air. The chilled water air-washer system also cools the air below its dew point by using water that is colder than the dew-point temperature. The water vapor in the air condenses on the water spray or the nearest surface. In this case, the use of colder water results in greater dehumidification. The type of system chosen for illustration in this article is the DX cooling coil system. The basic components of this mechanical refrigeration system are an evaporator coil, compressor, condenser, and throttling valve (or expansion valve). The system uses a refrigerant that enters the evaporator coil (cooling coil) in a liquid state. The refrigerant evaporates inside the coil and, in doing so, absorbs heat from the process air moving through the coil. It then leaves the coil in the form of a gas. The compressor takes the cold vapor from the evaporator and compresses it to a hot gas at high pressure. When the refrigerant leaves the compressor, it is still a gas but at a much higher pressure (five to ten times greater) and a much higher temperature. The hot refrigerant gas is then pushed through a condenser (in this case, an air-cooled condenser) where the hot gas is cooled and condensed into a liquid by some substance, usually air or water. The refrigerant then flows from the condenser as a high-pressure liquid through the expansion valve. As the liquid passes through the valve, its pressure is suddenly decreased to the pressure in the evaporator coil. At the same time, the temperature of the liquid refrigerant drops down from the warm condenser temperature to the cold evaporator temperature. This occurs because a small amount of liquid suddenly flashes to a vapor as it passes through the restriction in the valve. Then the liquid, with some bubbles of flash vapor, enters the evaporator coil. The liquid refrigerant in the coil evaporates and, in doing so, absorbs heat from the air passing through a coil. An example of a DX dehumidification system is illustrated in Fig. 1. The system includes a heat-pipe heat exchanger and an air-cooled condenser system. The heat pipe removes 538,616 Btu per hr (44.88 ton) of heat from the inlet process air and passes it to the dehumidifier process air leaving the system. The advantage is reduced cooling load and a leaving air condition that is not at or near saturation. The operating cost in Table 4 is $20.03 per 1000 lb of water vapor removed at a process-air-leaving condition of 56.3 F and 36 grains per lb (53.9 percent RH). Dehumidification reheat systems will use the high-pressure, high- temperature gas leaving the compressor in a coil system to reheat the process air leaving the dehumidifier. Most common residential dehumidifiers use this configuration. It is important for designers to consider waste energy usage, heat wheels, or heat pipes for reheat because reheat can add a significant "added" cost to the operating cost of a DX system.  Rotating Dry Desiccant WheelDry desiccants are adsorbent materials that attract moisture because of the electrical field at the desiccant surface. The field attracts water molecules that have a net opposite charge. Some of the solid adsorbents used in dry desiccant systems are illustrated in Table 5.Sorption is the adsorption process by which a desiccant removes water vapor directly from the air.  The ability of an adsorbent to attract
moisture depends on the difference
in vapor pressure between
the desiccant surface and air. The
vapor-pressure difference drives
moisture from the high vaporpressure
area to the low vaporpressure
area. Dry desiccants typically
have low vapor pressure at
their surface and, therefore, adsorb
moisture from the air. When
moisture is removed from the process
air stream, it produces heat
of sorption (or heat of adsorption),
which is composed of latent heat
of condensation of the removed
moisture plus additional chemical
heat. The heat of sorption of the
moisture removed from the air is
converted to sensible heat. The
amount of heat released is usually
around 1080 Btu per lb WV removed
to 1312 Btu per lb WV removed.
The actual amount depends
on the type of desiccant.
The heat of sorption (sensible
heat) is energy that is passed to
the process air stream, which
raises the discharge air temperature
of the process air stream. The ability of an adsorbent to attract
moisture depends on the difference
in vapor pressure between
the desiccant surface and air. The
vapor-pressure difference drives
moisture from the high vaporpressure
area to the low vaporpressure
area. Dry desiccants typically
have low vapor pressure at
their surface and, therefore, adsorb
moisture from the air. When
moisture is removed from the process
air stream, it produces heat
of sorption (or heat of adsorption),
which is composed of latent heat
of condensation of the removed
moisture plus additional chemical
heat. The heat of sorption of the
moisture removed from the air is
converted to sensible heat. The
amount of heat released is usually
around 1080 Btu per lb WV removed
to 1312 Btu per lb WV removed.
The actual amount depends
on the type of desiccant.
The heat of sorption (sensible
heat) is energy that is passed to
the process air stream, which
raises the discharge air temperature
of the process air stream. 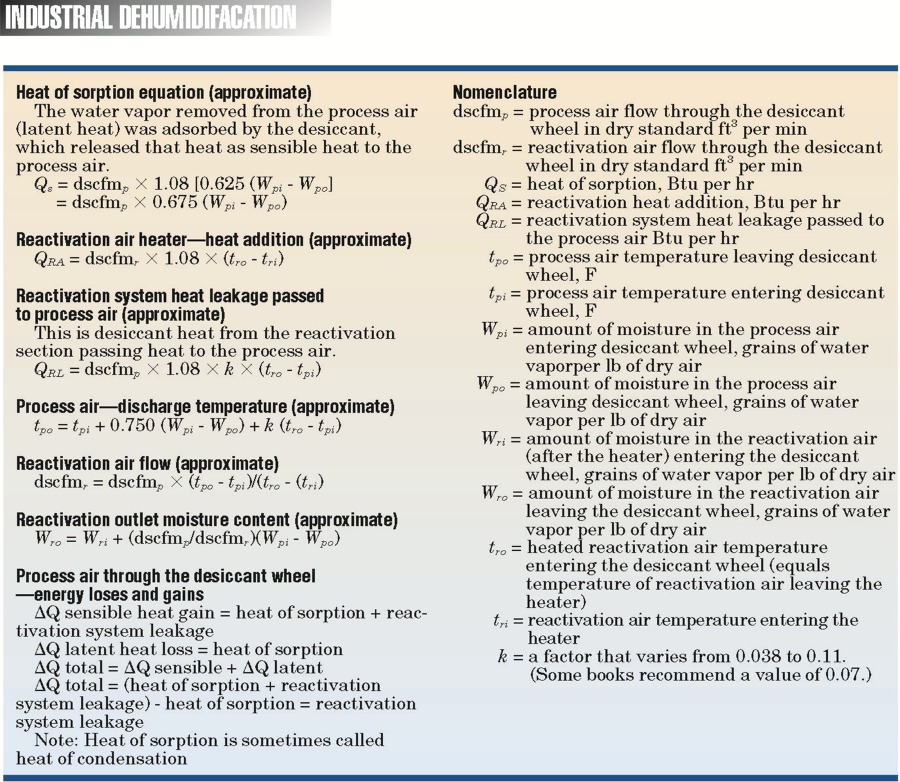 As the moisture content of the desiccant rises, so does the watervapor pressure at the desiccant surface. At some point, the vapor pressure at the desiccant surface will be the same as the air, and moisture adsorption will end. The desiccant is then taken out of the process air stream and is placed into the reactivation air stream (a scavenger air stream consisting of outside air or building air). The reactivation air stream is typically heated to a temperature of 190 to 375 F. The combination of the heat and moisture raises the vapor pressure at the desiccant surface. When the surface vapor pressure exceeds the vapor pressure of the reactivation air, moisture leaves the desiccant. This process is called reactivation. The reactivation section, which constitutes less than half of the desiccant wheel, uses flexible seals to seal it from the adsorption or process side to minimize cross contamination. The typical leakage rate is 1 to 2 percent. Following reactivation, the hot desiccant rotates back into the process air where the process air cools the desiccant which lowers the desiccant vapor pressure, so it can collect more moisture from the balance of the process air stream. Some of the equations used by the rotating dry desiccant manufacturers are shown in the accompanying sidebar. A typical layout of a rotary dry desiccant system is illustrated in Fig. 2. The operating costs of the system are listed in Table 6. The operating cost for the example in Fig. 1 is $15.87 per 1000 lb of water vapor removed with a leaving air condition of 93.6 F and 35 grains per lb. If the air is too hot and has to be cooled to 55 F and 35 grains per lb, the operating cost increases to $34.24 per 1000 lb WV. Reactivation heat represents 86 percent of the operating cost for the direct-fired unit and 88 percent for the indirect-fired unit. This represents a great opportunity for the designer to use lowcost hot water from cogeneration; the use of low-cost steam; or condensate, refrigeration reject heat, or waste exhaust to preheat the reactivation air. These options could significantly reduce the operating cost. Keep in mind that in some cases it may be more economical to combine cooling and desiccant dehumidification. The technologies do complement each other since the refrigeration condenser reject heat from the cooling process can be used to preheat the reactivation air. In process-drying applications, dry desiccant dehumidifiers are sometimes used without added cooling because the increase in temperature caused by the heat of adsorption is helpful in the drying process. However, in some applications, a provision must be made to remove the excess sensible heat from the process air after dehumidification. For this reason, Table 6 provides an added cost section for cooling the air to 55 F as an example of the possible added cost. Liquid Desiccant DehumidifierLiquid desiccant dehumidification operates on the principal of chemical absorption of water vapor from the air. The absorbent or desiccant solution will change physically, chemically, or both during the sorption process. Some of the liquid desiccant solutions used for dehumidification are: • Lithium chloride (LiCl) • Lithium chloride (LiCl)• Lithium bromide (LiBr) • Calcium chloride (CaCl2) • Triethylene glycol (TEG) • Propylene glycol Liquid absorption dehumidification is very similar to a chilled water air-washer system. When the air passes through the washer, its dew point approaches the temperature of the water supplied. Air that is more humid is dehumidified, and air that is less humid is humidified. In a similar manner, the liquid absorption dehumidifier sprays the air with a desiccant solution that has a lower vapor pressure than the vapor pressure of the entering process air stream. The liquid has a vapor pressure lower than water at the same temperature, and the air passing over the solution approaches this reduced vapor pressure. The ability to remove water vapor (or add water vapor) is determined by the temperature and concentration of the solution. The conditioner can be adjusted so that the conditioner delivers air at the desired relative humidity. The vapor pressure of a given concentration of absorbent solution approximates the vapor-pressure values of a fixed relative humidity line on a psychrometric chart. For instance, a 40 percent concentration of lithium chloride closely approximates the 20 percent relative humidity line. Also, a 15 percent concentration is very close to the 80 percent relative humidity line. Therefore, it can be said that higher solution concentrations give lower equilibrium relative humidity and thus allow the absorbent to dry air to lower levels. Temperature also affects the absorbents' ability to remove moisture. For instance, a 25 percent solution lithium chloride has a vapor pressure of 0.37 in Hg at 70 F (same as air at 70 F and 50 percent RH). When the solution is heated to 100 F, the vapor pressure climbs to 0.99 in Hg. Therefore, the warmer the desiccant, the less moisture it can absorb. Also, if the solution vapor pressure is higher than the surrounding air, the water vapor will transfer to the air and dry the desiccant solution. A typical system diagram is illustrated in Fig. 3. In the operation, warm, moist air is sprayed with a solution of chilled lithium chloride, which was cooled with chilled water in a plate-and-frame heat exchanger. The air is cooled and dehumidified by heat and mass transfer to the lithium chloride solution. A chiller with an air-cooled condenser section provides the chilled water to cool the lithium chloride solution. If the desired dehumidified air-moisture content is 50 grains per lb (or 48 F dew point), the water used to cool the desiccant can be 85 F cooling-tower water rather than chilled water. Consult Table 1 for additional information. When moisture is removed from the air, the reaction liberates heat. This is the reverse of evaporation, when heat is consumed by the reaction. The heat that is generated is the latent heat of condensation of the water vapor plus the heat of solution (or the heat of mixing of the water and desiccant). In desiccant dehumidification, this heat (approximately 1080 to 1320 Btu per lb water vapor removed) is transformed to the air, raising the air dry bulb temperature, and therefore, increasing the load on the chilled water system. The chilled water system must be sized to remove the latent heat of condensation (heat of sorption), the air sensible heat, and the residual heat load added by the regeneration process. To remove the water extracted from the air and keep the liquid desiccant at a fixed concentration, a small percentage of the conditioner- desiccant pump flow (typically around 15 percent) is transferred to the regeneration system. The weak desiccant solution is pumped up to a heating system (plate-and-frame heat exchanger), which raises the temperature and vapor pressure of the liquid desiccant. The hot desiccant is then sprayed at a scavenger air stream (outside air or building air) with a lower vapor pressure that forces the water vapor out of the desiccant and into this air, which is exhausted outside. The dry desiccant returns to the regenerator sump. The desiccant is still a little warm, and its vapor pressure is still a little high—until it flows back to the conditioner and is cooled by the chilled water heat exchanger. Therefore, the cooling system must be sized to include this residual heat load added by the regeneration process (sometimes called heat dump back). The amount of heat and dump back is typically in the range of 50 to 350 Btu per lb water vapor removed. Table 7 is a review of the operating costs associated with the system in Fig. 3. The total operating cost is $28.43 per 1000 lb of water vapor removed from the process air. You will note that the natural gas cost is 37 percent of the total cost. Therefore, it pays to find a source of waste heat to reduce these costs. Some possible sources are condenser-rejected heat, solar heat, or a natural gas engine-driven chiller, which produces hot water (engine heat) as well as chilled water at a reduced cost. The operating cost of a liquid desiccant dehumidification system combined with a natural gas engine-driven chiller is $19.85 per 1000 lb of water vapor removed. This combination represents a 30 percent reduction in operating energy costs. Another option to save energy on liquid desiccant systems is to install a liquid-to-liquid-type heat exchanger (some call this an interchanger) placed between the warm desiccant leaving the regenerator and the cool desiccant entering the regenerator. By doing so, less energy is needed to regenerate the desiccant because it is warmer than when it left the regenerator. The heat exchanger will typically reduce the heat dump back to the conditioner-cooling consumption by about 65 percent and reduces the regenerator heat consumption by about 15 percent. ConclusionTable 8 is a summary of the operating costs for each of the large, industrial dehumidifiers using the electricity cost of $0.06 per KWH and the natural gas cost of $5.00 per 106 Btu. One can see that the dry desiccant systems have the lowest energy operating cost of all the systems, but they also have the highest discharge air temperatures.If elevated process air temperature is not acceptable, the liquid desiccant or DX with heat pipe would be the choice (at 55 to 56 F discharge air temperature). The outcome of this study will change, depending upon the actual energy costs for that area. In the United States, natural gas costs vary from $2.40 to $7.90 per 106 Btu and electricity goes from $0.018 to $0.15 per KWH. What this says is that every system must be evaluated based on the energy costs for that region. Also, the evaluation of operating costs should include maintenance and capital amortization. 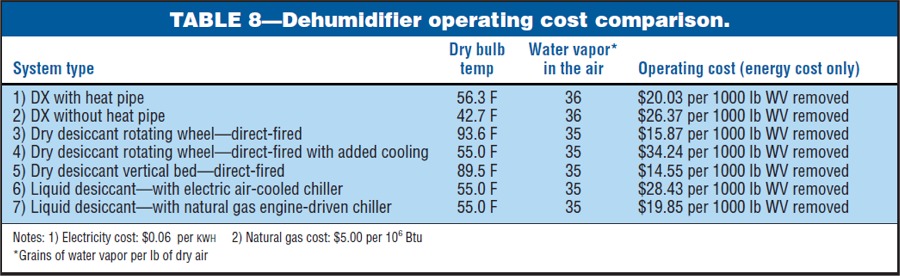 Each system is unique, and they all offer ways to reduce energy operating costs. For example, the liquid desiccant system operating cost dropped 30 percent (from $28.43 to $19.85 per1000 lb WV removed) by incorporating a natural gas engine-driven chiller and using the hot water jacket heat to preheat the regenerator system. Also if the required dew point is 48 F (49.68 grains per lb) or above, cooling-tower water can be used in the summer. The DX systems can use heat pipes, heat wheels, and/or heat reject from the process cooling for reheat. Using purchased energy for DX reheat can raise the operating costs significantly. The major operating cost for dry desiccant systems is the reactivation heat that represents 86 percent of the total operating cost for the system in Table 6. Using alternative low-cost energy sources can reduce the operating costs significantly. DX system condenser reject heat could be used to preheat the reactivation air as well as desuperheater coil heat. For this reason, the most economical system may be a combination DX system and desiccant (solid or liquid) system. The technologies do complement each other since the refrigeration condenser reject heat from the air cooling process can be used to prevent the air entering the reactivation or regeneration section of the desiccant dehumidifier. In industrial plants, there are many sources of cheap, low-grade heat (low temperature) that can be used in the reactivation or regeneration system. It is up to the engineer involved in the equipment selection to consider these sources before a decision can be made on the type of system. I would like to thank the following companies for assistance in the preparation of this article. Without their assistance, it would not have been possible: Kathabar, Des Champs Laboratories, Munters Cargocaire, Bry-Air, Inc., Desert Aire, Therma-Stor Products, Drieaz, Dectron, Inc. I would also like to thank Craig Pekarek for the CAD work and Jodi Cavil for the typesetting of this article. [ back to top ] Bibliography (Part 1) 1) "Water Vapor Migration and Condensation Control in Buildings," by W. Acker, Heating/Piping/Air Conditioning Magazine, June 1998. 2) Zimmerman, O.T., and I. Lavine, Industrial Research Services: Psychrometric Tables and Charts, 2nd Ed., Industrial Research Services, Inc., Dover, New Hampshire, 1964. Industrial Dehumidification Part 2:
|
|||
| Example 1: Air pressure = 29.921 in. Hg Air temperature = 70 F Relative humidity = 100 percent Humidity ratio = 109.93 grains WV per lb dry air Moist air density = 0.074190 lb wet air per cu ft wet air Specific volume = 13.6906 cu ft wet air per lb dry air acfm flow = 40,000 cu ft wet air per min Dehumidification industry dscfm = 38,956 dry std cu ft per min Fan industry scfm = 39,568 std cu ft per min |
Example 2: Air pressure = 29.921 in. Hg Air temperature = 200 F Relative humidity = 50 percent Humidity ratio = 2809 grains WV per lb dry air Moist air density = 0.051216 lb wet air per cu ft wet air Specific volume = 27.3599 cu ft wet air per lb dry air acfm flow = 40,000 cu ft wet air per min Dehumidification industry dscfm = 19,493 dry std cu ft per min Fan industry scfm = 27,315 std cu ft per min |
Example 1 shows that at 70 F, the dscfm flow is very close to the fan industry scfm flow (only 1.6 percent variation). However, at more elevated temperatures, such as in Example 2, the fan industry scfm flow is 40 percent higher than the dscfm flow.
Water Vapor Loads Due to Air Flow
Water vapor loads on industrial buildings come from many sources. Listed below are a few of these sources of water vapor:People
Permeation through walls, roofs, and floors
Moisture from products and packaging materials
Evaporation from open tanks or wet surfaces
Product dryer leakage
Open combustion
From air flow
• Air leakage through cracks and holes
• Air leakage through conveyor openings
• Intermittent door openings
• Building-to-building air infiltration
• Makeup air
In many cases, water vapor loads by air flow are a major contributor to the total building vapor load. In the research work for this article, I came across a number of approximate equations that are used to calculate the water vapor load from air flow. Approximate equations can be fairly accurate as long as the air conditions are close to 70 F air temperature.
[ back to top ]
Industrial Dehumidification Part 3: Boiler & Plant Operations
by William G. Acker
Accurate water vapor load equations for industrial dehumidification systems design are difficult to find, and terminology is not standard. This article provides a thorough review of both.
The first article in this series, which covered air flow, can be read in HPAC Engineering's May 1999 issue. WATER VAPOR LOADS DUE TO AIR FLOW Water vapor loads on industrial buildings come from many sources. Listed below are a few of these sources of water vapor:
❒ People
❒ Permeation through walls, roofs, and floors
❒ Moisture from products and packaging materials
❒ Evaporation from open tanks or wet surfaces
❒ Product dryer leakage
❒ Open combustion
❒ Air flow
• Air leakage through cracks and holes
• Air leakage through conveyor openings
• Intermittent door openings
• Building-to-building air infiltration
• Makeup air
In many cases, water vapor loads by air flow are a major contributor to the total building vapor load. In the research work for this article, I came across a number of approximate equations that are used to calculate the water vapor load from air flow. Approximate equations can be fairly accurate as long as the air conditions are close to 70 F air temperature. For more information on water vapor permeation loads, consult the June 1998 issue of HPAC Engineering. The next few sections will compare approximate and exact equations for selected water vapor sources. MOISTURE FROM AIR LEAKAGE Equations (8) to (12) can be used to calculate moisture for air leakage through cracks, holes, and conveyor openings. Equations (8) to (11) were taken from engineering books or from manuals prepared by dehumidification companies. Notice that the engineering units do not properly cancel out, which is why they are considered approximate equations.
Equations (11) and (12) are applied and compared in the example below to show how results from approximate and exact equations can vary under different conditions. Equation (11), which is approximate, calculates a water vapor load 20.18 percent over the exact equation (12). Equation (11) is more accurate if the entering air flow is close to 70 F. Engineers preferring the exact equation will need a psychrometric chart to obtain the entering air specific volume, or a psychrometric computer program that can calculate the air mixture properties.
Example conditions:
 Room conditions
Room conditionsAir pressure: 29.921 in. Hg
Dry bulb temp: 70 F
Moisture level: 35 grains WV/lb dry air
Relative humidity: 32.38 percent
Entering air flow conditions
Air pressure: 29.921 in. Hg
Dry bulb temp: 120 F
Moisture level: 420 grains WV/lb dry air
Relative humidity: 76.44 percent
Specific volume: 16.02379 cu ft wet air/lb dry air
Air flow acfm: 200 cu ft per min

Equations (13) and (14). In this example, equations (13) and (14) produced water vapor loads that were 7.27 and 12.63 percent above the exact equation (12) water vapor load. The error is a direct result of the assumed entering air specific volume. Note that the makeup air specific volume will vary with the entering air psychrometric properties. Therefore, you cannot select a standard value for specific volume and expect the equation to be exact. For this reason, equations (13) and (14) are approximate equations. Equations (13) and (14), which can be found in many engineering books and dehumidification manuals, will be fairly accurate as long as the entering makeup air is close to the selected specific air volume.
Example Conditions
 Inside room conditions
Inside room conditionsAir pressure: 29.921 in. Hg
Dry bulb temperature: 70 F
Moisture level: 35 grains WV/lb dry air
Relative humidity: 32.38 percent
Entering makeup air
Air pressure: 29.921 in. Hg
Dry bulb temperature: 100 F
Moisture level: 280 grains WV/lb dry air
Relative humidity: 93.67 percent
Specific volume: 15.01722 cu ft wet air/lb dry air
Air flow acfm: 2000 cu ft per min

Water Vapor Removed by Dehumidifiers
Dehumidification systems remove water vapor from the process air that travels through the unit. This section looks at the equations used to determine the humidity ratio of the process air entering and leaving the unit as well as equations used to estimate the amount of water vapor removed when the inlet and discharge humidity ratios and air flow are known. Note that some of the equations are the same as the equations used in preceding sections. The equations listed as approximate can be very accurate if the process air flow temperature is close to 70 F (Table 1). In this series of equations and calculations, approximate equations (15) and (11) produced water vapor removals that were 40 and 105 percent above the exact equations (16) and (17). The approximate equations can be fairly accurate if the air entering the dehumidifier is close to 70 F. Engineers that desire greater accuracy can use the psychrometric chart to get the specific volume needed to make the conversion from acfm to dscfm or purchase psychrometric programs that can calculate the value for them.
Conclusion
Many of the flow diagrams presented in articles, books, engineering manuals, and proposals from dehumidification companies do not indicate the engineering units for the flows in the diagrams. As indicated in this article, the flows are illustrated in cfm or scfm with no explanation. In most cases, the flows are in dry standard cubic feet per minute.Over the years, I have been contacted by many engineers over the issue of calculated water vapor load variances from different equations. In most cases, the approximate equations are equations that have been shortened to make the calculations easier for engineers. If you have any questions on your equations, check the engineering units to make sure that they properly cancel out to grains of water vapor per hr, or lb of water vapor per hr.
The author would like to thank his wife, Sandra, for her patience and assistance during the preparation of these articles. He would also like to thank Nels Strand, the author's mentor and close friend for over 20 years.
[ back to top ]
Bibliography (Part 3)
1) Acker, W., Water Vapor Migration and Condensation Control in Buildings, HPAC Engineering, June 1998.
2) Acker, W., Industrial Dehumidification Water Vapor Load Calculations and System Descriptions, HPAC Engineering, March 1999.
3) Clifford, G. E., Modern Heating and Ventilating Systems Design, Prentice Hall, Englewood Cliffs, N.J., 1992.
4) Fan Engineering, 8th Ed., Edited by Robert Jorgensen, Buffalo Forge, Buffalo, N.Y., 1983.
5) 1997 ASHRAE Handbook: Fundamentals, American Society of Heating, Refrigerating, and Air-Conditioning Engineers, Inc., Atlanta, Ga.

 Dehumidification manufacturers
like to develop air flow diagrams of their entire systems. Mass flow analysis is used in these
diagrams because mass flow does not change if there is a
temperature or pressure change. Mass flows can also be
added and subtracted. The actual air flows or acfm (cu ft
per min) will change if the temperature or pressure
changes. Also, acfm values cannot be added or subtracted
because they are at different air densities. The
dehumidification industry chose a type of mass flow
analysis that is flow in dry standard cu ft per min (dscfm)
at a common air density of 0.075 lb dry air per dry standard
cu ft. The acfm flows are converted to these flows
for illustration in the air system diagrams (shown in Fig.
1 and Fig. 2).
Dehumidification manufacturers
like to develop air flow diagrams of their entire systems. Mass flow analysis is used in these
diagrams because mass flow does not change if there is a
temperature or pressure change. Mass flows can also be
added and subtracted. The actual air flows or acfm (cu ft
per min) will change if the temperature or pressure
changes. Also, acfm values cannot be added or subtracted
because they are at different air densities. The
dehumidification industry chose a type of mass flow
analysis that is flow in dry standard cu ft per min (dscfm)
at a common air density of 0.075 lb dry air per dry standard
cu ft. The acfm flows are converted to these flows
for illustration in the air system diagrams (shown in Fig.
1 and Fig. 2). As mentioned earlier, dscfm air flow is a flow at a common
air density of 0.075 lb dry air per dry standard cu ft. I prefer to use the term dscfm to avoid any possible confusion
with acfm or the fan industry term scfm. In Fig. 1,
one manufacturer uses cfm to represent the dry standard
air flow. When you look at this diagram, it is easy to tell
that the flows are dry standard air flows and not acfm. If
you look at the flow in and out of the dehumidifier, the
flows are both 7500 cfm. This is not possible with acfm
because this flow is made of both air and water vapor;
therefore, there is a loss (of water vapor or cfm) as it travels
through the dehumidifier. Listed below is an example
of an acfm flow broken down into dry air flow and water
vapor flow:
As mentioned earlier, dscfm air flow is a flow at a common
air density of 0.075 lb dry air per dry standard cu ft. I prefer to use the term dscfm to avoid any possible confusion
with acfm or the fan industry term scfm. In Fig. 1,
one manufacturer uses cfm to represent the dry standard
air flow. When you look at this diagram, it is easy to tell
that the flows are dry standard air flows and not acfm. If
you look at the flow in and out of the dehumidifier, the
flows are both 7500 cfm. This is not possible with acfm
because this flow is made of both air and water vapor;
therefore, there is a loss (of water vapor or cfm) as it travels
through the dehumidifier. Listed below is an example
of an acfm flow broken down into dry air flow and water
vapor flow:
